NTI sets up expert panel after TGA meeting
Today, our biotech Investment, Neurotech International (ASX: NTI) established an expert advisory group to provide guidance on its PANDAS/PANS drug development program following productive discussions with the Australian Therapeutic Goods Administration (TGA).
We see this is a positive development as the TGA has indicated a potential provisional registration pathway for NTI's lead drug candidate NTI164, which could accelerate approval by up to 2 years.
This is important as it would provide line of sight on the commercialisation of NTI’s treatment, not just for PANDAS/PANS, but potentially for Rett Syndrome and ASD as well.
This is important as NTI estimates the annual market for PANDAS/PANS is worth US$1.2 billion - providing access to a treatment in Australia via TGA provisional registration would be a major milestone for NTI and an excellent outcome for PANDAS/PANS patients.
Particularly after the positive outcome of the Phase I/II PANDAS/PANS trial which was the first of multiple (3) successful clinical trials for NTI:

NTI’s clinical trial meets primary endpoints for neurological disease
An entry into the Australian market would provide important bona fides to potential institutional investors, and we hope, a precursor to a breakthrough into more lucrative markets such as the US.
From small things, big things grow…
Earlier this month, the clinical trial results for PANDAS/PANS got even better - with the treatment showing continued improvement well after meeting primary endpoint:
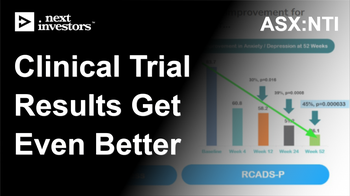
NTI’s PAN/PANDAS clinical trial results get even better
Unfortunately, despite multiple successful clinical trials, the NTI share price is languishing a bit at 7.2 cents after an impressive run that started well in advance of its string of impressive clinical trial results.
But as NTI’s commercialisation strategy becomes clearer, and the regulatory pathways to registration approach, the monetisation of the treatment should become more real for investors and the market more generally.
With regards to registration, a provisional registration from the TGA, if approved, could save up to 2 years of development time for NTI164, with the overall process taking ~12-18 months.
That would be a major bonus and we remain long term holders of NTI…
How does this news impact our NTI Investment Memo?
Why did we invest in NTI?
NTI is targeting “orphan” diseases - treatments that are highly valuable
NTI is currently targeting PANDAS/PANS and Rett Syndrome, which are potentially eligible for orphan drug designation. The average price for an orphan drug is US$32,000 per year per patient and government approvals to market are fast tracked.
We see this as a major part of our Investment Thesis for NTI - and the company is making good progress on this after today’s news.
The development of novel treatments for rare neurological disorders, particularly in paediatric populations, is an area of high unmet medical need and growing interest from biopharmaceutical companies.
Regulatory agencies like the FDA and TGA have implemented various pathways and incentives to encourage the development of orphan drugs for these underserved patient populations.
With its focus on rare paediatric neurological disorders like PANDAS/PANS, Rett syndrome, and we think NTI is well-positioned to capitalise on this macro trend.
The company's unique cannabinoid formulation NTI164 has delivered strong clinical results and could offer a differentiated treatment option for these conditions where few approved therapies exist.
🎓Learn: Orphan Drugs Explained
What’s next for NTI?
🔄Provisional registration application
NTI expects to finalise its preferred clinical development plan and select the target indication (PANDAS/PANS, Rett syndrome, or autism) for a potential provisional registration application in early Q3 2024.
The company will continue to work closely with its regulatory and clinical advisors, including the newly established expert advisory group, to build consensus and drive awareness of PANDAS/PANS as it prepares for potential regulatory submissions.
🔄Cerebral palsy trial
NTI will also likely continue to advance its broader clinical development program for NTI164 in other neurological indications, with a Phase I/II trial in spastic cerebral palsy already approved.
That’s just a couple things to look forward to for us in what will be an extremely busy and high newsflow year for NTI:
Metabologenomic data from Phase I/II PANDAS/PANS Clinical Trial
Orphan Drug Designation Europe - Rett Syndrome
Orphan Drug Designation Europe - PANDAS/PANS
Orphan Drug Designation USA - Rett Syndrome
Orphan Drug Designation USA - PANDAS/PANS
Completion of Patient Recruitment Phase I/II Cerebral Palsy
Commence Phase I/II Cerebral Palsy Trial
FDA IND/EMA toxicology
Presentation of Phase I/II Rett Syndrome data at international Rett Syndrome conference






